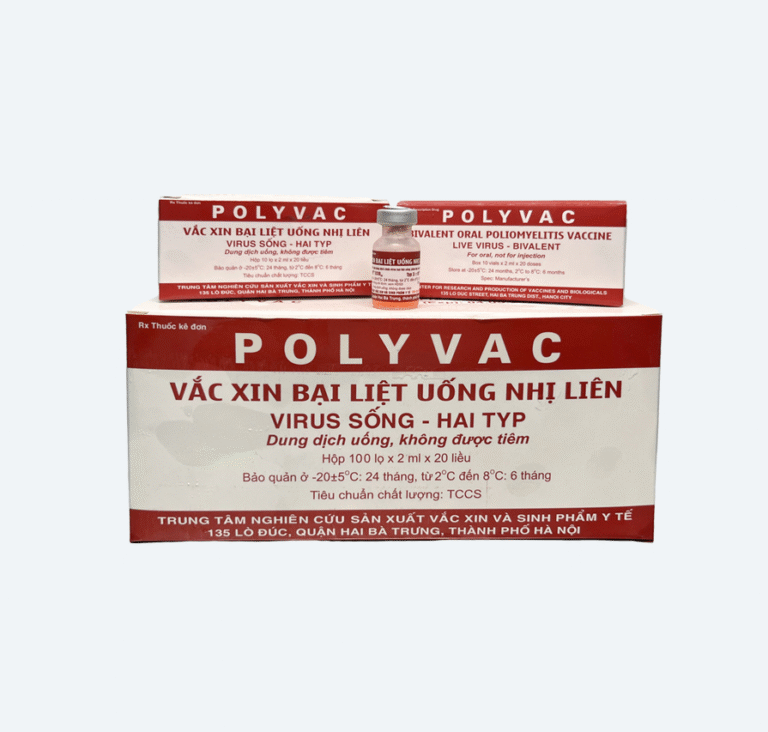
Bivalent Oral Polio Vaccine (bOPV)
Consists of live attenuated types 1 and 3 viruses (Sabin strain) produced on Macaca mulatta monkey kidney cells and stabilized with Magnesium Chloride. The vaccine complies with Vietnamese and World Health Organization standards.
- Polio virus Type 1: ≥10⁶ʰ⁰ CCID₅₀
- Polio virus Type 3: ≥10⁵ʰ⁵ CCID₅₀
- Dosage: 0.1ml/dose (2 drops)
- Target group: Children aged 6-12 weeks
- Dosing schedule: At least 3 doses, 4 weeks apart
- Route: Oral (do not inject)
- Antibiotics: Kanamycin, Erythromycin
- Storage: -20±5°C (24 months) or 2-8°C (6 months)
- Packaging: 10 doses/vial or 20 doses/vial
- Contraindications: Immunodeficiency, vomiting, diarrhea
Product Information
This section provides detailed information on ingredients, dosage, administration, indications, and important considerations when using bivalent oral polio vaccine (bOPV). You will find complete instructions on storage, contraindications, adverse reactions, and necessary warnings to ensure safety and efficacy during immunization.
Each 0.1ml dose (equivalent to 2 drops) contains:
- Attenuated Polio virus:
- Type 1: ≥10⁶ʰ⁰ CCID₅₀
- Type 3: ≥10⁵ʰ⁵ CCID₅₀
- Color indicator: Phenol red
- Stabilizer: Magnesium Chloride
- Antibiotics: Kanamycin, Erythromycin
Clear pink solution.
Polio vaccine is indicated for active immunization against Polio.
The target subjects are infants aged 6 – 12 weeks according to the EPI schedule, all non-immunized children up to 18 years old, and high-risk adults. However, adults should use inactivated polio vaccine (IPV).
Oral route: Vaccine is for oral use only (do not inject).
Oral dose: 0.1ml/dose (equivalent to 2 drops)
Administration Schedule: According to the EPI schedule for children from 2 months of age. Children must take at least 3 doses of polio vaccine, each dose separated by at least 4 weeks.
Administration Method: To thaw the vaccine, use a dropper to withdraw the vaccine and directly drop two drops into the child's mouth.
Note: After use, vaccine vials and droppers must be collected into medical waste containers and disposed of according to medical waste regulations.
Do not use for children with leukemia, lymphoma, and other malignancies; cellular immunodeficiency or absence of cellular immunity (hypogammaglobulinemia or agammaglobulinemia); those on immunosuppressants; vomiting; diarrhea.
Individuals with a history of allergy to any component of the vaccine.
Vaccination should be deferred for acute high-fever illnesses, severe chronic diseases, or acute infections with fever.
Avoid child's saliva contamination of the dropper. Children with diarrhea can receive the vaccine but should be re-vaccinated the month after the diarrhea stops.
Caution should be used in individuals allergic to other vaccine components.
Caution when using concurrently with cholera, typhoid, or plague vaccines. After taking oral polio vaccine, limit contact with immunocompromised individuals for 4-6 weeks.
Immunocompromised people should limit close contact with those who recently received OPV for 4-6 weeks.
Pregnancy Period
Vaccination is not recommended during pregnancy, although use during pregnancy does not harm the fetus or placenta (no increase in fetal death, natural miscarriage, or higher congenital defects compared to controls). If absolutely necessary, OPV can be given to pregnant women.
Lactation Period
Breast milk contains antibodies against polio virus indirectly related to the mother's serum titer.
When administering polio vaccine to newborns during breastfeeding, polio antibodies, highest in colostrum, can interfere with the vaccine's immune response. Thus, to prevent efficacy loss, breastfeeding should be paused for 6 hours before and after vaccination.
There is no evidence of the vaccine's effect on the ability to drive or operate machinery.
Interaction: Can be used concurrently with Diphtheria-Tetanus vaccine, DTP vaccine, BCG vaccine, Measles vaccine, Rubella vaccine, Mumps vaccine, and Yellow Fever vaccine.
Incompatibility: Must not be combined with Typhoid vaccines.
Oral polio vaccine should not be used by those on immune-affecting medications like steroids, anti-cancer drugs, radiation therapy, or those who have undergoing major surgery or are severely exhausted.
Polio vaccine administration may reduce the reaction to the tuberculin test.
No reports of adverse vaccine reactions have been recorded.
Adverse reactions are very rare (ADR 1/1,000,000).
No cases of Polio caused by the vaccine have been detected during its circulation.
No side effects were observed in sick children when receiving the vaccine.
Report any adverse reactions to your doctor when using the vaccine.
Overdose: There are no data on vaccine overdose; do not exceed the specified vaccine dose.
Management of drug overdose: Actively monitor to have timely management measures.
Pharmacodynamics
ATC Code: J07BF04
Mechanism of action: Oral polio vaccine stimulates the immune system to produce antibodies against polio virus types 1 and 3. The vaccine virus persists in the digestive tract for 4-6 weeks, no longer capable of spreading to the central nervous system, stimulating neutralizing antibody production and humoral immunity.
Antibody stimulation begins 7-10 days after OPV administration, peaking at 3 weeks. Most children are protected after dose 1, and the vast majority after 2 doses. Studies in children show 95% of vaccine recipients have protective antibodies for both virus types for 5 years post-immunization.
Vaccines must be stored at -20 ± 5°C until the expiry date on the vial. After thawing, the vaccine should be kept at 2 - 8°C for no longer than 6 months.
Opened vaccine vials must be used within the day and stored at 2 - 8°C.
24 months from manufacture date when stored at -20 ± 5°C.
6 months from manufacture date when stored at 2 - 8°C.
Institutional standard.
01 vial contains 1ml of vaccine solution (equivalent to 10 doses) or 2ml (equivalent to 20 doses).
One small box contains 10 vaccine vials with an instruction leaflet.


